
Cervical Cancer Screening for Individuals at Average Risk:2020 Guideline Update from the American Cancer Society
ACS:美国癌症学会
Abstract: The American Cancer Society (ACS) recommends that individuals with a cervix initiate cervical cancer screening at age 25 years and undergo primary human papillomavirus (HPV) testing every 5 years through age 65 years (preferred); if primary HPV testing is not available, then individuals aged 25 to 65 years should be screened with cotesting (HPV testing in combination with cytology) every 5 years or cytology alone every 3 years (acceptable) (strong recommendation). The ACS recommends that individuals aged >65 years who have no history of cervical intraepithelial neoplasia grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening (qualified recommendation). These new screening recommendations differ in 4 important respects compared with the 2012 recommendations: 1) The preferred screening strategy is primary HPV testing every 5 years, with cotesting and cytology alone acceptable where access to US Food and Drug Administration-approved primary HPV testing is not yet available; 2) the recommended age to start screening is 25 years rather than 21 years; 3) primary HPV testing, as well as cotesting or cytology alone when primary testing is not available, is recommended starting at age 25 years rather than age 30 years; and 4) the guideline is transitional, ie, options for screening with cotesting or cytology alone are provided but should be phased out once full access to primary HPV testing for cervical cancer screening is available without barriers. Evidence related to other relevant issues was reviewed, and no changes were made to recommendations for screening intervals, age or criteria for screening cessation, screening based on vaccination status, or screening after hysterectomy. Follow-up for individuals who screen positive for HPV and/or cytology should be in accordance with the 2019 American Society for Colposcopy and Cervical Pathology risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors.
摘要:
美国肿瘤学会(ACS)推荐从25岁开始至65岁,进行每5年一次的宫颈癌HPV初筛,如果无法进行HPV初筛(primary HPV tests),则可以每5年进行一次细胞学和HPV联合检查,或每3年进行一次细胞学检查。对于年龄大于65岁的,如果在过去的25年内没有CIN2及以上病变,或过去10年内有充分证据表明是阴性的筛查结果,则可停止筛查。相比于2012年的指南,这份指南有4点变化。1.宫颈癌筛查策略首选初筛HPV检查,对于难以获得FDA批准的初筛HPV检测,联合筛查或单独细胞学检查也是可以接受的。2.首次筛查的年龄由21岁变为25岁。3.初筛HPV检查以及联合筛查或单独细胞学检查的开始年龄为25岁,而不是30岁。4.本指南将做为过渡期使用,也就是说,联合筛查和单独细胞学检查目前是可接受的,但是初筛HPV得到普及时,这两种检查将被逐步替代并淘汰。
我们对与其他相关问题的证据进行了审查,对筛查间隔时间、年龄或停止筛查的标准、基于疫苗接种状态的筛查或子宫切除术后筛查的建议未作修改。对HPV和/或细胞学阳性的人群的随访仍遵循2019年美国阴道镜及病理学会(ASCCP)对于宫颈癌筛查异常结果的处理指南。

美国癌症协会宫颈癌筛查建议,2020年
本指南适用于所有正常宫颈人群,不论其性生活史以及疫苗接种情况如何,包括接受宫颈上子宫切除术以及保留宫颈的变形男性。
本指南代表了美国癌症协会(ACS)对那些开始进行子宫颈癌筛查,或在过去有过正常子宫颈癌筛查结果,或根据风险管理建议已经回归常规宫颈癌筛查的人群的管理指南。这些建议不适用于因实体器官移植或干细胞移植、人体免疫缺陷病毒感染或其他原因造成的免疫抑制、或在子宫内暴露于己烯雌酚而导致换宫颈癌风险增加的人群。
建议:
25-65岁女性首选每5年通过初筛HPV来筛查宫颈癌。如果无法进行初筛HPV检查(primary HPV tests),则可以每5年进行一次细胞学和HPV联合检查,或每3年进行一次细胞学检查。
由于在某些情况下,难以获得FDA批准的初筛HPV检测,联合检测或细胞学检查也是可接受的。随着美国向HPV单独初筛的过渡,在未来的指南中,联合检测或细胞学筛查将被逐渐替代。
对于年龄大于65岁的,如果在过去的25年内没有CIN2及以上病变,或过去10年内有充分证据表明是阴性的筛查结果,则可停止筛查。
充分证据的阴性指的是,过去10年内连续2次的HPV阴性结果、或连续2次的联合筛查阴性结果,或连续3次的细胞学阴性结果,按照推荐的筛查间隔进行筛查。本指南不适用于因宫颈筛查结果异常所进行的监测。
对于年龄大于65岁的人群,没有足够的筛查结果,则应持续筛查至满足终止筛查的条件。
任何年龄预期寿命有限的人群,都可终止筛查。
Page 3
Evolution in Cervical Cancer Prevention and Early DetectionOn the basis of accumulated evidence and assessment of the balance of benefits and harms, in 2018, the US Preventive Services Task Force (USPSTF) included stand-alone HPV testing (primary HPV testing) among the tests recommended for cervical cancer screening.
Currently, there are only 2 FDA-approved primary HPV tests available for cervical cancer screening,14,16,17 and both are approved for primary HPV testing beginning at age 25 years (Table 3).14 Five HPV tests are FDA-approved for cotesting. Although it is too early to measure utilization of primary HPV testing for cervical cancer screening, utilization of cotesting has increased, whereas screening with cytology alone has declined.
宫颈癌预防和早期筛查的演变
根据已有证据的以及权衡利弊后,2018年,美国预防服务工作组在宫颈癌筛查中加入了HPV单独检查(HPV初筛)。
目前,只有2种FDA批准的初筛HPV检测可用于宫颈癌筛查。二者都被批准用于25岁开始的宫颈癌筛查(表3)。有5种FDA批准的联合筛查HPV检测产品。虽然,评价HPV初筛的应用还为时过早,但是联合筛查的应用在逐渐增加,相应的,单独细胞学检测在逐渐减少。


Page 4-6
Methos 方法
The ACS volunteer Guideline Development Group (GDG) is responsible for developing cancer screening guidelines following a protocol that is designed to maintain rigor, transparency, independence, and consistency.
For the update of the cervical cancer screening guideline, the GDG chose to use 2 reports commissioned by the USPSTF for its 2018 cervical cancer screening update as sources of evidence to inform recommendations: 1) a systematic evidence review on cervical screening conducted by the Kaiser Permanente Research Affiliates Evidence-Based Practice Center,4,43 and 2) a decision analysis based on a mathematical disease-simulation model that was produced by researchers at the Center for Health Decision Science of the Harvard T.H. Chan School of Public Health.44,45 The ACS staff conducted continued surveillance of the literature on cervical cancer screening outcomes and reviewed potentially relevant articles after the publication date of the evidence review report (August 2018).
Factors in Developing Recommendations
1. The balance between desirable and undesirable effects:
2. The quality of evidence:
3. Values and preferences:
方法
ACS指南推进小组(GDG)负责推进指南的更改,并遵循严谨、公开、独立和一致性原则。GDG小组分析了建模得出的系统的证据以及补充证据。考虑了筛查策略的利与弊以及患者自身的干扰因素。分析评估了不同筛查策略的优劣。
GDG小组共选择了2018年由美国预防工作组(USPSTF)宫颈癌筛查指南中引用的已报道的2篇文献:1.凯撒医疗中心进行的宫颈癌筛查的系统性数据回顾。2.哈佛大学T.H.陈公共卫生学院健康决策科学中心进行的基于数学疾病模拟模型的决策分析。ACS的工作人员对宫颈癌筛查的相关文献进行了持续的追踪调查,并收集了相关报道(2018年8月)。
影响指南制定的因素
1. 预期与非预期结果之间的平衡:,
2. 证据的质量
3. 患者的价值观和偏好
Page 8 宫颈癌筛查的初始年龄
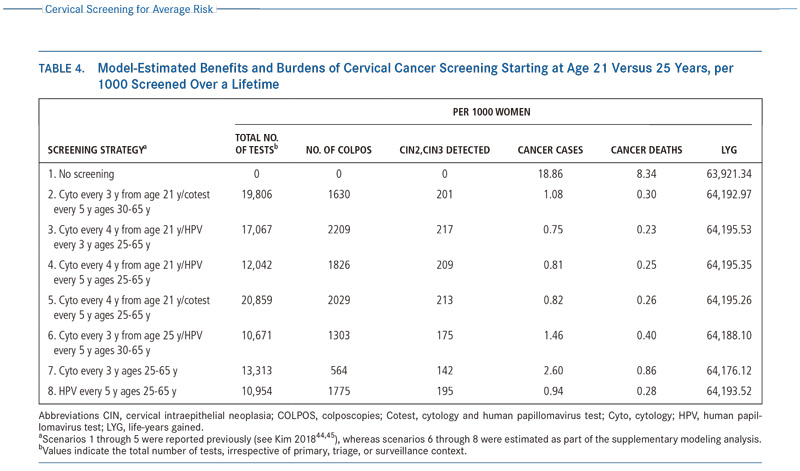
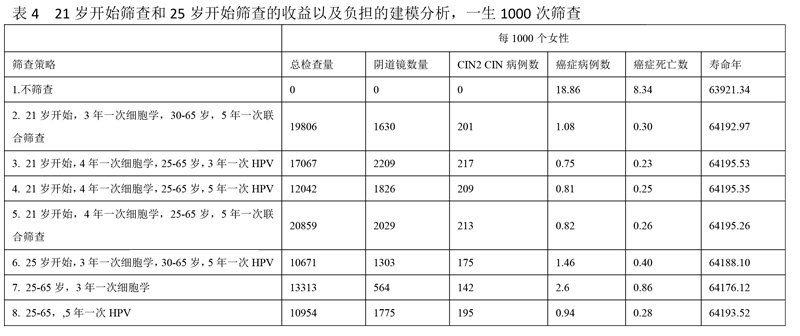
In the supplemental modeling analysis (Table 4),44,45 starting screening with primary HPV testing at age 25 years, compared with a screening strategy of cytology alone from age 21 years followed by switching to primary HPV testing at age 25 years, retained >99% of the life-years gained, (64,193 vs 64,195, respectively) with fewer colposcopies (1775 vs 1826). Also, compared with the strategy of cytology alone beginning at age 21 years and switching to cotesting at age 30 years, starting screening with primary HPV testing at age 25 years showed a 13% gain in cervical cancer cases prevented and a 7% gain in cervical cancer deaths prevented, with similar life-years gained (64,193 vs 64,194, respectively) and with only 9% more colposcopies but 45% fewer tests (HPV or cytology) required overall. Furthermore, it is expected that the colposcopy rate will decline as HPV vaccination coverage expands and a growing fraction of HPV-vaccinated women reach the age to begin screening.18
在建模补充分析中(表4),从25岁开始宫颈癌HPV初筛,相比于21岁开始进行单独细胞学筛查,在25岁时转为HPV初筛,保留了99%的寿命年(分别是64193和64195),阴道镜的检查数量相对减少(分别是1775和1826)。同样,比较了在21岁开始单独细胞学筛查,在30岁开始联合筛查,在25岁开始宫颈癌HPV初筛多预防了13%的宫颈癌以及7%的死亡,同样的寿命年,只增加了9%的阴道镜检查量,但是总体减少了45%的检查量(不论是HPV还是细胞学)。此外,随着HPV疫苗接种量的扩大,以及越来越多的HPV疫苗接种者达到了宫颈癌筛查的年龄,阴道镜的检查数量还将减少。
Page 10
Screening Strategies for Cervical Cancer
The evidence from RCTs75,76,97-99 and other studies100,101 showed that HPV-based cervical cancer screening has superior sensitivity and long-term negative predictive value compared with cytology-alone screening. Data from a routine screening practice in a large US health system show that the risk of future CIN3+ and cervical cancer declines with an increasing number of negative cotests.100 The low risk of subsequent cancer conferred by a negative HPV test result was similar to that for HPV alone and cotesting.100,101The FDA indication for each of the 2 assays approved for primary HPV screening states that women who test negative for hrHPV types should be followed according to the physician’s assessment of medical and screening history, other risk factors, and professional guidelines.14 The clinical trials on which the FDA based its approval of tests for HPV primary screening had a follow-up time of only 3 years.74,102 However, since 2012, cotesting has been recommended at a 5-year interval.5 For primary HPV screening, with similar benefits and lower risk of harms, guidelines from leading organizations, including those from the ACS,5 ASCCP,5 the American Society for Clinical Pathology,5 USPSTF,15 and the American College of Obstetricians and Gynecologists103 recommend a screening interval of 5 years based on evidence from a wider range of studies and the results of microsimulation modeling.
A recent trend analysis showed a continued increase inadenocarcinoma incidence rates.2 Given the known limitations of cytology for adenocarcinoma detection,3 several studies have suggested that screening with HPV testing could improve the detection of adenocarcinoma and its precursors,75,104-106 although the systematic evidence review judged the evidence to be uncertain.4
宫颈癌筛查策略
随机对照实验以及其他相关研究表明,相比于细胞学筛查,HPV筛查具有较高的敏感性以及较长时间的阴性预测价值。来自美国卫生系统的大型筛查数据表明,联合筛查使得人群未来患CIN3+以及宫颈癌的风险降低。FDA对2种被批准用于初筛HPV检查的检测方法的指示表明,高危HPV阴性的人群应根据医生对医疗和筛查史、其他危险因素和专业指南的评估进行随访。FDA批准的HPV初筛临床试验进行了3年的随访。然而,自2012年开始,联合筛查就已经被推荐采用5年筛查间隔。初筛HPV检查,介于HPV初筛具有与联合检查相同的效益,更少的伤害,以及对于微观仿真建模的大量数据结果的收集分析,ACS\ASCCP\USPSTF以及美国妇产科医师协会一致推荐5年一次的HPV筛查间隔。
最近的趋势分析表明,宫颈腺癌的检出率在逐渐升高。由于细胞学检查对于腺癌筛查的敏感度有限,一些研究表明,HPV检查,可以提高腺癌及其前期的检测,尽管系统性的证据还未被确认。
The microsimulation modeling analysis conducted for the USPSTF suggests that, compared with no screening, screening with cytology alone every 3 years beginning at age 21 years, cotesting every 5 years after switching at age 30 years from cytology alone, and primary HPV testing every 5 years after switching at age 30 years from cytology alone, with screening ceasing at age 65 years under all strategies, can reduce the number of cervical cancer deaths from 8.34 (no screening) to 0.76, 0.30, and 0.29 deaths per 1000 women, respectively.44,45 Compared with cytology alone, the higher CIN detection associated with primary HPV testing is accompanied by an increased number of false-positives and likely more colposcopies. In contrast, cytology alone has lower sensitivity for precancer and cancer than primary HPV testing or cotesting.4 In the decision analysis,44,45 cytology alone resulted in the lowest benefit in terms of life-years gained and cancer cases prevented and the lowest number of CIN2 or CIN3 and CIN 3+ cases detected.44,45 Because of the higher number of total tests, cotesting was not efficient across any of the screening measures. For these reasons, the USPSTF concluded that cotesting was an alternative to the preferred strategies of primary HPV testing every 5 years and cytology alone every 3 years.15 The evidence indicates that primary HPV testing is more effective compared with cytology alone and is more efficient than cotesting.
美国预防工作组(USPSTF)的微观仿真模型分析建议,相比于不筛查,21岁开始的3年一次的细胞学筛查,30岁开始转为5年一次的联合筛查,或30开始转为每5年一次的HPV筛查,直至65岁停止筛查。分别能够减少宫颈癌的死亡数由每1000人8.34(不筛查)至0.76, 0.30和0.29。相比于单独细胞学筛查,HPV初筛,CIN的检出率越高,伴随着的假阳性率以及阴道镜的检查数量也越多。相反,相较于HPV初筛和联合筛查,细胞学对于癌症以及癌前病变的敏感性较低。在决策分析中,细胞学检查在预防癌症以及延长寿命方面的收益最低,并且,检查出的CIN2、CIN3以及CIN3+的病例数最少。因为总体检查数量较多,联合筛查并不是最有效率的。介于以上原因,USTFPF工作组提出,联合筛查可作为5年一次的单独HPV初筛及3年一次的单独细胞学筛查的备选方案。证据表明,HPV初筛相比于细胞学检查更为有效,相比于联合筛查效率更高。
Page 17 Future Directions
Primary HPV Testing and the Diminishing Role of Cytology Screening
Despite known limitations in accuracy and quality-assurance challenges, the burden of cervical cancer, particularly squamous cell cervical cancer, has been successfully reduced since the middle of the last century in populations with widespread access to cytology. However, the development of molecular assays for detecting HPV is an advance in cervical cancer screening that offers improved sensitivity and better reassurance of low future cancer risk from a negative screening test for a causative agent compared with cytology.Primary HPV testing was not included as an option for screening in the 2012 ACS guideline update due to several factors, including lack of an FDA-approved test for primary screening and because the evidence for the effectiveness of primary HPV testing in the majority of studies was limited to a single round of screening.5 Since 2012, several RCTs have been published on the effectiveness of primary HPV testing for cervical cancer screening that include subsequent rounds of screening,43 and there are now FDA-approved tests for primary screening. Although the FDA approved the first test for primary HPV screening in 2014, as of 2017, only 40.6% percent of laboratories reported that they offered primary HPV screening, and, among those that did, primary HPV screening was a very small fraction of all HPV-associated testing.134 Furthermore, the survey reported variability in the settings where HPV genotyping (for management of positive results, not primary HPV screening) was available.
未来的方向
HPV初筛的应用、细胞学筛查作用的逐渐削弱
尽管细胞学检查在精确度和质量上有所欠缺,但是也正是由于上世纪中叶细胞学的广泛使用,使得宫颈癌,特别是宫颈鳞癌的发病率得到降低。然而,分子生物学HPV病原体的检测技术的发展,提供了一种更为敏感的,更能够保证阴性人群安全的宫颈癌筛查方法。在2012年的ACS指南中,并没有包括HPV初筛策略,是由于几个因素,包括FDA还未批准任何一种初筛HPV试剂,对于HPV筛查的有效性验证证据不足,仅限于单轮筛查等。2012年开始,越来越多的采用HPV初筛有效性的多轮随机对照筛查试验得以发表,现在,已有FDA批准的初筛HPV试剂。尽管,FDA在2014年就批准了首个HPV初筛产品,直至2017年,只有40.6%的实验室报道其能够提供HPV 初筛,并且,HPV初筛只占所有HPV相关检测的一小部分。此外,调查显示,大多数实验室所提供的HPV分型检测(其对阳性结果的处理,不适用于作为初筛HPV检测)缺乏规范。
下载: 2020年ACS指南 全文.pdf
2020年ACS指南 全文.pdf